Unsweetened coconut, mayonnaise, sour cream, blue
cheese salad dressing, almonds, pecans, olives, avocados,
and sausages—what do all these foods have in common?
More than 50% of the kilocalories in each of these food
items comes from fat, a vital nutrient in our diet.
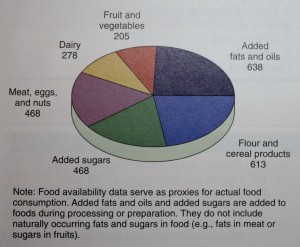
As shown below added fats and oils provide more
kilocalories in the average American’s diet than any other
food group. Examination of food supply trends in the United
States indicates an increase in added fat intake with a greater
portion of the fat coming from vegetable fats, whereas satu-
rated fat and cholesterol intake has decreased. Consumer
concerns about healthy food choices explain these changes.
Food manufacturers, producers, and grocers have responded by:
(1) trimming fat from meats
(2) providing leaner cuts of beef and pork
(3) replacing tropical oils in processed foods
(4) manufacturing foods containing less fat
In addition, consumers have:
(5) increased their consumption of fish and poultry
(6) substituted lower fat milk for whole milk
The fat content of very lean beef and pork cuts currently com-
pares favorably with a skinless chicken breast.
CLASSIFICATION
Fats in the diet should actually be called lipids. Lipids
contain the same three elements as carbohydrates:
Lipids contain less oxygen in proportion to hydrogen and carbon than
carbohydrates. Because of their structure, they provide more energy per
gram than either carbohydrates or proteins.
The two classes of water-insoluble substances are
(1) simple lipids or triglycerides, which occur in foods and in
the body
(2) structural lipids, which are produced by
the body for specific functions.
The structural component of lipids is fatty acids.
Triglycerides with at least one of the fatty acids
replaced with carbohydrate, phosphate, or nitrog-
enous compounds are called compound lipids. Dietary
lipids usable by the body include triglycerides, fatty acids,
phospholipids, and cholesterol. Lipoproteins are found
solely in the body.
Added fats and oils provide more calories per day
for the average American than any other food group in 2006.
(Data from the U.S. Department of Agriculture and Economic
Research Service.)
CHEMICAL STRUCTURE
Triglycerides are composed of fatty acids and glycerol, as
shown:
Monoglycerides = glycerol + one fatty acid
Diglycerides = glycerol + two tatty acids
Triglycerides = glycerol + three fatty acids
A fatty acid is a chain of carbon atoms attached to hydro-
gen atoms with an acid grouping on one end. Glycerol is the
alcohol portion of a triglyceride to which the fatty acids
attach. Triglycerides are the most common fat present in
animal or protein foods.Monoglycerides and
diglycerides are found in the small intestine and result from
the breakdown of triglycerides during digestion. Free
fatty acids, monoglycerides, and glycerol can cross cell
membranes.
All three fatty acids attached to the triglyceride can be
different: they can be long, medium, or short, and saturated
or unsaturated. Medium-chain and short-chain fatty acids
are readily digested and absorbed, but most fats in foods
(especially vegetable fats) contain predominantly long-chain
fatty acids. Short-chain fatty acids contain less than six
carbon atoms, medium-chain fatty acids contain 6 to 10
carbon atoms, and long-chain fatty acids contain 12 or
more carbon atoms.
SATURATED FATTY ACIDS
Fatty acids are classified according to their degree of satura-
tion. Saturation of a fatty acid depends on the number of
hydrogen atoms attached to the carbon. Saturated fatty
acids (SFAs) contain only single bonds, with each carbon
atom having two hydrogen atoms attached to it.
Palmitic and stearic acids are the two most prevalent SFAs.
They are structural components of tooth enamel and
dentin.
MONOUNSATURATED FATTY ACIDS
When adjacent carbon atoms are joined by a double bond
because two hydrogen atoms are lacking, there is a gap
between the hydrogen atoms in the chain; the fatty acid is
unsaturated.Monounsaturated fatty acids (MUFAs)
contain only one double bond. The most abundant
MUFA is oleic acid. Oleic acid is also a structural
component of the tooth.
TRANS FATTY ACIDS
Hydrogenation is a commercial process in which vegetable
oil is converted to a solid margarine or shortening by adding
hydrogen to the oil. This process results in naturally unsatu-
rated vegetable oils being changed to an SFA by changing
unsaturated bonds to saturated bonds. Hydrogenation can be
controlled, so “tub” or “soft” margarine is “partially hydro-
genated.” or not completely saturated. The hydrogenation
process not only increases the proportion of SFAs, but also
the shape of the fatty acid. When the hydrogen atoms are
rotated so that they are on opposite sides of the bond, in the
“trans” position, the fatly acid is called atrans fatty acid.
Partial hydrogenation results in large numbers of
fatty acids having this altered shape. Foods with trans fatty
acids have a longer shelf life, and flavors are stable. The
most common trans fatty acid is elaidic acid, found in par-
tially hydrogenated vegetable oils, such as tub margarines
and cooking oils. A naturally occurring trans fatty acid with
double bonds on adjacent carbons is present in small amounts
in milk and meat of ruminants (cows, sheep, and deer).
POLYUNSATURATED FATTY ACIDS
When numerous carbons in a fatty acid are connected
by double bonds, the fatty acid is polyunsaturated. A
polyunsaturated fatty acid (PUFA) has two or more double
bonds. Linoleic, arachidonic, and a conjugated
linoleic acid are PUFAs. These PUFAs are omega-6 fatly
acids. They have their first double bond on the sixth carbon
from the omega (terminal) end; they are also referred to as
n-6 PUFAs.
PUFAs naturally occur in what is called the “cis”
configuration (i.e., the carbon chain bends so that hydrogens
stick out on the same side of the molecule). These trans
PUFAs, called conjugated linoleic acid (CLA), have
naturally occurring double bonds on adjacent carbons.
CLAs are a derivative of linoleic acid and have different
physiological functions than commercially produced trans
fatty acids.
Omega-3 fatty acids, or α -linolenic acids, make up
another class of PUFAs. These fatty acids are unique in that
the first double bond is located three carbon atoms from the
omega end of the molecule; hence they are called omega-3’s
or n-3’s. Omega-3 fatty acids include α-linolenic acid,
which has 18 carbon atoms and two double bonds,
eicosapentaenoic acid (EPA), which has 20 carbon atoms
and five double bonds.
CHARACTERISTICS OF FATTY ACIDS
The carbon chain length and degree of saturation determine
various properties of fats, including their flavor and hardness
or melting point (the temperature at which a product
becomes a liquid). Most SFAs are solid at room temperature;
because most animal fats are predominately saturated fats,
they are solid at room temperature. Short-chain fatty acids
(12 carbon atoms or less), MUFAs, and PUFAs that are
liquid at room temperature are called oils. Milk fat contains
a large amount of short-chain SFAs.
Fats with a high proportion of unsaturated fatty acids may
deteriorate or become rancid, resulting in unpleasant flavors
and odors. Fats become rancid when subject to high tem-
peratures and exposure to light, which cause oxidation and
decomposition of fats. The decomposition results in perox-
ides that may be toxic in large amounts. Vitamin E, a fat-
soluble vitamin, is an antioxidant and, to some degree,
protects the oil to which it is added; however, in doing so,
vitamin E is inactivated so that it cannot be used by the body.
Other antioxidants, such as butylated hydroxyanisole (BHA)
and butylated hydroxytoluene (BHT), are added to com-
mercially processed fats and oils to prevent their spoilage.
to be continued…